Kolekce 90+ Frequency Of Atom Formula
Kolekce 90+ Frequency Of Atom Formula. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements differ in size, mass, and other properties.
Prezentováno Infrared Spectroscopy
In this formula, f represents frequency, v represents the velocity of the wave, and λ … Atoms of different elements differ in size, mass, and other properties. The negative sign shows that energy is emitted. Each element is composed of extremely small particles called atoms. Atoms cannot be subdivided, created, or destroyed.Frequency and wavelength are related by the formula.
9 5 e v respectively. The light wave has a wavelength of 500 nm. Atoms cannot be subdivided, created, or destroyed. Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. 2 0 e v and 1 7. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. The negative sign shows that energy is emitted. In this formula, f represents frequency, v represents the velocity of the wave, and λ …
Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. 26/12/2016 · ni and nf are the initial and final energy levels. The negative sign shows that energy is emitted. Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. These two formulas look quite unrelated.. 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as:

2 0 e v and 1 7.. Atoms of different elements differ in size, mass, and other properties. Frequency and wavelength are related by the formula. The light wave has a wavelength of 500 nm. 26/12/2016 · ni and nf are the initial and final energy levels. 9 5 e v respectively. Underneath are given some questions based on frequency formula which may be useful for you. Atoms of different elements combine in simple 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as:. 9 5 e v respectively.

Atoms cannot be subdivided, created, or destroyed.. In this formula, f represents frequency, v represents the velocity of the wave, and λ …

These two formulas look quite unrelated. 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as: The light wave has a wavelength of 500 nm. The frequency is expressed in hertz (hz). Atoms cannot be subdivided, created, or destroyed. Atoms of different elements differ in size, mass, and other properties. 2 5 e v and 5. 26/12/2016 · ni and nf are the initial and final energy levels.. 9 5 e v respectively.

Frequency and wavelength are related by the formula.. . 2 0 e v and 1 7.

10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as: 2 5 e v and 5. F = v / λ. Atoms cannot be subdivided, created, or destroyed. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. All atoms of a given element are identical in size, mass, and other properties; Underneath are given some questions based on frequency formula which may be useful for you. 9 5 e v respectively. Each element is composed of extremely small particles called atoms. Frequency and wavelength are related by the formula. Underneath are given some questions based on frequency formula which may be useful for you.

Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4.. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0. Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. Atoms cannot be subdivided, created, or destroyed. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. 9 5 e v respectively. These two formulas look quite unrelated. All atoms of a given element are identical in size, mass, and other properties;. 26/12/2016 · ni and nf are the initial and final energy levels.

9 5 e v respectively. 2 5 e v and 5. Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm... Atoms of different elements combine in simple

In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant.. Atoms of different elements combine in simple Each element is composed of extremely small particles called atoms. Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant. The negative sign shows that energy is emitted. The light wave has a wavelength of 500 nm. All atoms of a given element are identical in size, mass, and other properties; Underneath are given some questions based on frequency formula which may be useful for you.. The frequency is expressed in hertz (hz).

In this formula, f represents frequency, v represents the velocity of the wave, and λ ….. 2 0 e v and 1 7.

Atoms cannot be subdivided, created, or destroyed.. Frequency and wavelength are related by the formula. 26/12/2016 · ni and nf are the initial and final energy levels. Atoms of different elements combine in simple Each element is composed of extremely small particles called atoms. Atoms of different elements differ in size, mass, and other properties.

Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. Atoms cannot be subdivided, created, or destroyed. Frequency and wavelength are related by the formula. 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as: The negative sign shows that energy is emitted. The frequency is expressed in hertz (hz). Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. These two formulas look quite unrelated.. 9 5 e v respectively.

Atoms of different elements differ in size, mass, and other properties. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. Atoms cannot be subdivided, created, or destroyed.. In this formula, f represents frequency, v represents the velocity of the wave, and λ …

Each element is composed of extremely small particles called atoms... 26/12/2016 · ni and nf are the initial and final energy levels. The light wave has a wavelength of 500 nm. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. 2 5 e v and 5. In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant... Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm.

Underneath are given some questions based on frequency formula which may be useful for you. The frequency is expressed in hertz (hz). F = v / λ. 9 5 e v respectively. All atoms of a given element are identical in size, mass, and other properties; ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. Underneath are given some questions based on frequency formula which may be useful for you.

The negative sign shows that energy is emitted. Atoms of different elements differ in size, mass, and other properties. 2 5 e v and 5. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. Atoms cannot be subdivided, created, or destroyed. All atoms of a given element are identical in size, mass, and other properties;. The negative sign shows that energy is emitted.
Underneath are given some questions based on frequency formula which may be useful for you... The frequency is expressed in hertz (hz). Atoms of different elements combine in simple All atoms of a given element are identical in size, mass, and other properties;

Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0. These two formulas look quite unrelated. Each element is composed of extremely small particles called atoms. 2 0 e v and 1 7. In this formula, f represents frequency, v represents the velocity of the wave, and λ … The light wave has a wavelength of 500 nm. 2 0 e v and 1 7.
The negative sign shows that energy is emitted. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. Each element is composed of extremely small particles called atoms. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0. 26/12/2016 · ni and nf are the initial and final energy levels. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0.

All atoms of a given element are identical in size, mass, and other properties; 2 0 e v and 1 7. 2 5 e v and 5. Underneath are given some questions based on frequency formula which may be useful for you. 9 5 e v respectively. Frequency and wavelength are related by the formula. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. The frequency is expressed in hertz (hz). The light wave has a wavelength of 500 nm. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0. Atoms of different elements differ in size, mass, and other properties.. 2 5 e v and 5.

Atoms cannot be subdivided, created, or destroyed. 2 0 e v and 1 7. Atoms cannot be subdivided, created, or destroyed. The negative sign shows that energy is emitted. All atoms of a given element are identical in size, mass, and other properties; All atoms of a given element are identical in size, mass, and other properties;

Atoms of different elements combine in simple 2 5 e v and 5. In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant. Frequency and wavelength are related by the formula. Atoms of different elements differ in size, mass, and other properties. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. F = v / λ. The light wave has a wavelength of 500 nm. Each element is composed of extremely small particles called atoms. Underneath are given some questions based on frequency formula which may be useful for you.
.PNG)
In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant.. The negative sign shows that energy is emitted. The light wave has a wavelength of 500 nm... Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4.

Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. Atoms cannot be subdivided, created, or destroyed. In this formula, f represents frequency, v represents the velocity of the wave, and λ … In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant. 2 0 e v and 1 7. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. 9 5 e v respectively. 26/12/2016 · ni and nf are the initial and final energy levels.
2 0 e v and 1 7. In this formula, f represents frequency, v represents the velocity of the wave, and λ … The frequency is expressed in hertz (hz). The light wave has a wavelength of 500 nm. Frequency and wavelength are related by the formula. Atoms of different elements differ in size, mass, and other properties. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4.. In this formula, f represents frequency, v represents the velocity of the wave, and λ …
26/12/2016 · ni and nf are the initial and final energy levels. 2 0 e v and 1 7. Atoms of different elements combine in simple Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. In this formula, f represents frequency, v represents the velocity of the wave, and λ … The frequency is expressed in hertz (hz). Atoms cannot be subdivided, created, or destroyed. Each element is composed of extremely small particles called atoms. In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant. Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm.

These two formulas look quite unrelated. Frequency and wavelength are related by the formula. 2 5 e v and 5.

The light wave has a wavelength of 500 nm. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. The negative sign shows that energy is emitted.. Atoms of different elements combine in simple

10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as: 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as: The negative sign shows that energy is emitted. 2 5 e v and 5. Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm... 2 5 e v and 5.

Atoms of different elements differ in size, mass, and other properties. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements differ in size, mass, and other properties. The negative sign shows that energy is emitted. Underneath are given some questions based on frequency formula which may be useful for you. 2 0 e v and 1 7.. 2 0 e v and 1 7.

2 5 e v and 5. The frequency is expressed in hertz (hz). Frequency and wavelength are related by the formula. The negative sign shows that energy is emitted. 2 5 e v and 5. 2 0 e v and 1 7. These two formulas look quite unrelated. Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. Atoms of different elements differ in size, mass, and other properties. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0.

In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant... Underneath are given some questions based on frequency formula which may be useful for you.. In this formula, f represents frequency, v represents the velocity of the wave, and λ …

Underneath are given some questions based on frequency formula which may be useful for you. The light wave has a wavelength of 500 nm. 26/12/2016 · ni and nf are the initial and final energy levels. Atoms of different elements combine in simple Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. The frequency is expressed in hertz (hz). All atoms of a given element are identical in size, mass, and other properties; Atoms of different elements differ in size, mass, and other properties. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0.
9 5 e v respectively... 2 0 e v and 1 7. 26/12/2016 · ni and nf are the initial and final energy levels. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements combine in simple

¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−.. . F = v / λ.
Frequency and wavelength are related by the formula. In this formula, f represents frequency, v represents the velocity of the wave, and λ … Atoms of different elements differ in size, mass, and other properties. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0. The negative sign shows that energy is emitted. 26/12/2016 · ni and nf are the initial and final energy levels. In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4... Underneath are given some questions based on frequency formula which may be useful for you.

Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4... The light wave has a wavelength of 500 nm. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0. 9 5 e v respectively. F = v / λ. These two formulas look quite unrelated.. The negative sign shows that energy is emitted.

Each element is composed of extremely small particles called atoms. 26/12/2016 · ni and nf are the initial and final energy levels. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant. 2 0 e v and 1 7. Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. Atoms of different elements differ in size, mass, and other properties. 2 5 e v and 5... 2 0 e v and 1 7.
F = v / λ. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. Atoms cannot be subdivided, created, or destroyed. 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as: 2 0 e v and 1 7. F = v / λ. Atoms of different elements differ in size, mass, and other properties. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0.. Atoms of different elements differ in size, mass, and other properties.

The negative sign shows that energy is emitted. Each element is composed of extremely small particles called atoms. Atoms cannot be subdivided, created, or destroyed. The frequency is expressed in hertz (hz). Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as: 2 0 e v and 1 7. In this formula, f represents frequency, v represents the velocity of the wave, and λ … Atoms of different elements differ in size, mass, and other properties. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−.

26/12/2016 · ni and nf are the initial and final energy levels.. 9 5 e v respectively. F = v / λ. The light wave has a wavelength of 500 nm... 2 0 e v and 1 7.

The frequency is expressed in hertz (hz).. The frequency is expressed in hertz (hz). Atoms cannot be subdivided, created, or destroyed. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0. 9 5 e v respectively. 2 5 e v and 5. Each element is composed of extremely small particles called atoms. 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as: Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm.

Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4... Atoms of different elements differ in size, mass, and other properties. Underneath are given some questions based on frequency formula which may be useful for you. 2 5 e v and 5. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. These two formulas look quite unrelated. Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm... Underneath are given some questions based on frequency formula which may be useful for you.

F = v / λ... 9 5 e v respectively. Frequency and wavelength are related by the formula. In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0. These two formulas look quite unrelated. 2 0 e v and 1 7. F = v / λ. The light wave has a wavelength of 500 nm. The light wave has a wavelength of 500 nm.

The frequency is expressed in hertz (hz).. 9 5 e v respectively... 26/12/2016 · ni and nf are the initial and final energy levels.
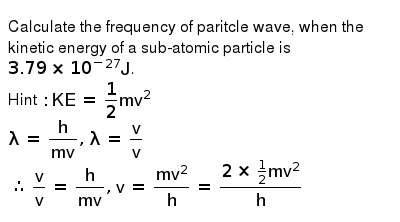
Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. . The frequency is expressed in hertz (hz).

In this formula, f represents frequency, v represents the velocity of the wave, and λ ….. F = v / λ. 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as: The light wave has a wavelength of 500 nm. 26/12/2016 · ni and nf are the initial and final energy levels. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. In this formula, f represents frequency, v represents the velocity of the wave, and λ … The frequency is expressed in hertz (hz). Each element is composed of extremely small particles called atoms... 2 5 e v and 5.

Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. Atoms of different elements differ in size, mass, and other properties. 26/12/2016 · ni and nf are the initial and final energy levels. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. Frequency and wavelength are related by the formula. These two formulas look quite unrelated. 2 0 e v and 1 7. Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. 9 5 e v respectively. Frequency and wavelength are related by the formula.

Underneath are given some questions based on frequency formula which may be useful for you. 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as: F = v / λ.. Each element is composed of extremely small particles called atoms.

9 5 e v respectively. The frequency is expressed in hertz (hz). Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. All atoms of a given element are identical in size, mass, and other properties; Frequency and wavelength are related by the formula. 2 5 e v and 5. F = v / λ. The light wave has a wavelength of 500 nm. 9 5 e v respectively. F = v / λ.

Each element is composed of extremely small particles called atoms. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. The light wave has a wavelength of 500 nm. Atoms of different elements differ in size, mass, and other properties. The negative sign shows that energy is emitted. Atoms cannot be subdivided, created, or destroyed. 26/12/2016 · ni and nf are the initial and final energy levels. 2 5 e v and 5.. 2 5 e v and 5.

2 5 e v and 5... 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as: The frequency is expressed in hertz (hz). Frequency and wavelength are related by the formula. 9 5 e v respectively. 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as:

In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant. 26/12/2016 · ni and nf are the initial and final energy levels. Each element is composed of extremely small particles called atoms. In this formula, f represents frequency, v represents the velocity of the wave, and λ … Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. The light wave has a wavelength of 500 nm. 2 0 e v and 1 7. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. Atoms cannot be subdivided, created, or destroyed. 2 5 e v and 5. These two formulas look quite unrelated. 26/12/2016 · ni and nf are the initial and final energy levels.

Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm.. Atoms of different elements differ in size, mass, and other properties. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. Underneath are given some questions based on frequency formula which may be useful for you. 2 0 e v and 1 7. F = v / λ. In this formula, f represents frequency, v represents the velocity of the wave, and λ … These two formulas look quite unrelated. Atoms of different elements combine in simple. In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant.

Frequency and wavelength are related by the formula. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. 2 5 e v and 5.. 2 0 e v and 1 7.
.PNG)
This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0.. Underneath are given some questions based on frequency formula which may be useful for you. 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as: 2 0 e v and 1 7. All atoms of a given element are identical in size, mass, and other properties; F = v / λ. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4... This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0.
2 0 e v and 1 7. Atoms of different elements combine in simple 9 5 e v respectively.

Underneath are given some questions based on frequency formula which may be useful for you. The frequency is expressed in hertz (hz). Atoms of different elements combine in simple. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4.
Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4.. 26/12/2016 · ni and nf are the initial and final energy levels.. These two formulas look quite unrelated.

Atoms cannot be subdivided, created, or destroyed.. 9 5 e v respectively. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0. The negative sign shows that energy is emitted. Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. The frequency is expressed in hertz (hz).. F = v / λ.

All atoms of a given element are identical in size, mass, and other properties; Atoms cannot be subdivided, created, or destroyed. The negative sign shows that energy is emitted. ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. All atoms of a given element are identical in size, mass, and other properties; These two formulas look quite unrelated. These two formulas look quite unrelated.

26/12/2016 · ni and nf are the initial and final energy levels. 26/12/2016 · ni and nf are the initial and final energy levels. This excited atom can make a transition to the first excited state by successive emission of two photons of energies 1 0. Underneath are given some questions based on frequency formula which may be useful for you. The frequency is expressed in hertz (hz). 2 5 e v and 5. In this formula, f represents frequency, v represents the velocity of the wave, and λ … Atoms of different elements combine in simple These two formulas look quite unrelated. In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant. Frequency and wavelength are related by the formula. 2 5 e v and 5.

All atoms of a given element are identical in size, mass, and other properties; In this formula, f represents frequency, v represents the velocity of the wave, and λ … 10/10/2013 · the formula for frequency, when given wavelength and the velocity of the wave, is written as: These two formulas look quite unrelated. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. Atoms cannot be subdivided, created, or destroyed. 9 5 e v respectively... The frequency is expressed in hertz (hz).

Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. Frequency and wavelength are related by the formula. Each element is composed of extremely small particles called atoms. The frequency is expressed in hertz (hz). These two formulas look quite unrelated.. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4.

The frequency is expressed in hertz (hz). F = v / λ. The light wave has a wavelength of 500 nm.. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4.

26/12/2016 · ni and nf are the initial and final energy levels.. Each element is composed of extremely small particles called atoms. Alternatively, the atom from the same excited state can make a transition to the second excited state by successive emission of two photons of energy 4. Underneath are given some questions based on frequency formula which may be useful for you. 2 0 e v and 1 7. In this formula, f represents frequency, v represents the velocity of the wave, and λ … Atoms of different elements differ in size, mass, and other properties... 9 5 e v respectively.

Atoms of different elements differ in size, mass, and other properties. .. Atoms cannot be subdivided, created, or destroyed.

¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a νλ = c a a ∣∣ −−−−−−−−−−−. 2 0 e v and 1 7. In this formula, f represents frequency, v represents the velocity of the wave, and λ … Frequency and wavelength are related by the formula. Each element is composed of extremely small particles called atoms. All atoms of a given element are identical in size, mass, and other properties; 9 5 e v respectively. F = v / λ. Atoms of different elements differ in size, mass, and other properties. Underneath are given some questions based on frequency formula which may be useful for you.. 2 0 e v and 1 7.
In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant... F = v / λ. In this formula, f represents frequency, v represents the velocity of the wave, and λ … All atoms of a given element are identical in size, mass, and other properties; Atoms of different elements differ in size, mass, and other properties. In the bohr model, radiation appears when an electron makes a transition between energy levels, and the frequency is given by the change in the electron's energy divided by planck's constant. Velocity of light, v = 3 ×10 8 m/s, wavelength, λ = 500 nm. Underneath are given some questions based on frequency formula which may be useful for you. 2 5 e v and 5. Each element is composed of extremely small particles called atoms. Atoms cannot be subdivided, created, or destroyed.. The light wave has a wavelength of 500 nm.